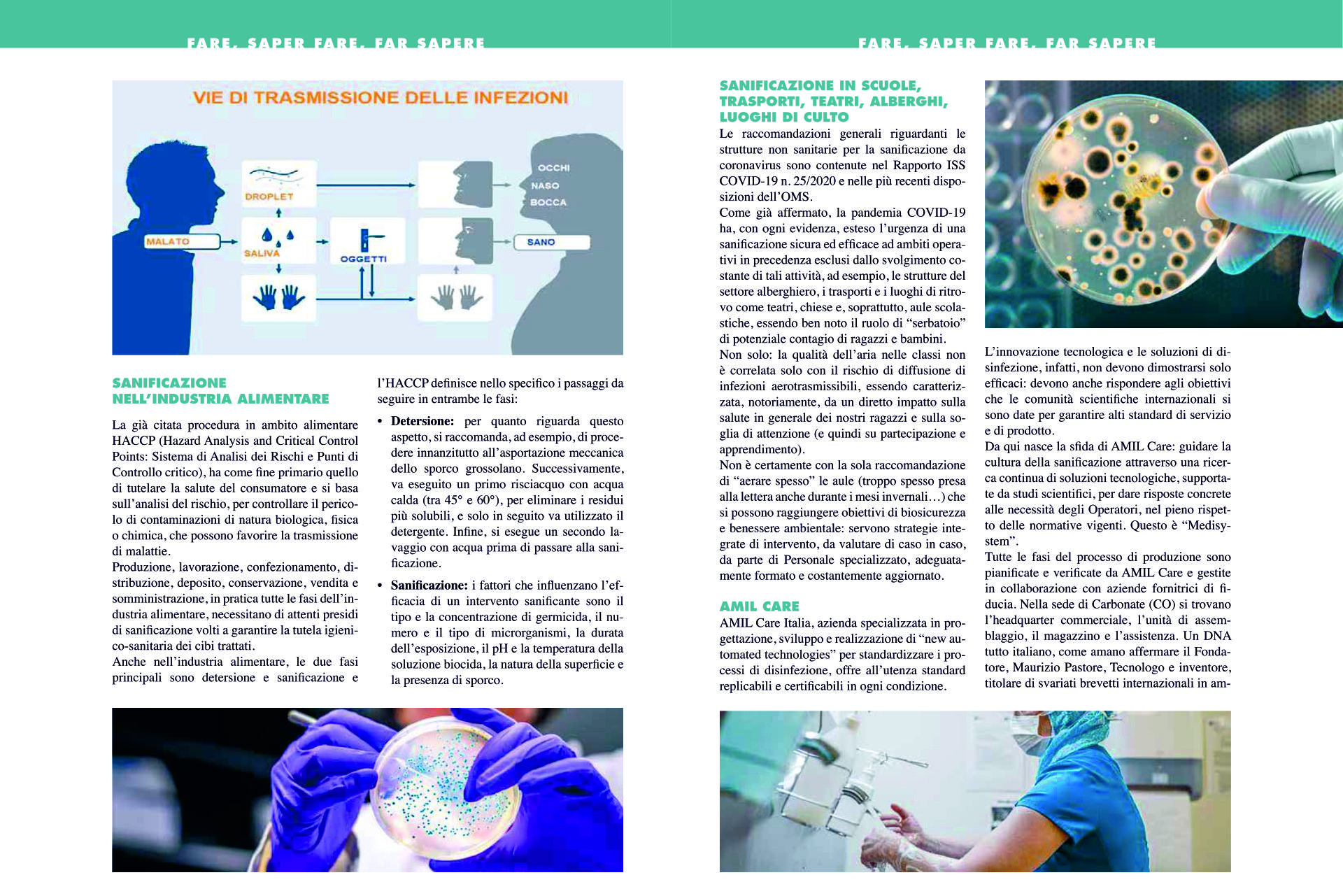
The optimal
high level
Bio-Disinfection
system
Hospital-acquired infections (HAIs) develop during healthcare practice, being a serious worldwide problem with an important cause of morbidity and mortality. HAIs are caused by various microorganisms and pathogens, which exhibit multidrug resistance and virulence.
In 2017, the World Health Organization (WHO) published a global list of antibiotic-resistant bacterias that deserve priority in new drug research. In this list the so-called ESKAPE bacterias occupy the first position, classified as critical priority (A. baumannii, P. aeruginosa, K. pneumoniae and Enterobacter) and high priority (E. faecium and S. aureus).
Microbial resistance was listed as one of the ten threats to global health in 2019. Approximately 700 thousand people die each year from infections caused by resistant pathogens, and it is believed that by 2050 that number will reach 10 million deaths.
Common types of HAIs include:
- Catheter-associated urinary tract infections
- Surgical site infections
- Bloodstream infections
- Clostridium difficile
- Pneumonia
AMIL Care’s aim to prevent HAIs and drive the culture of disinfection based on scientific studies in full compliance with current safety, plus local and international health regulations standards like GMP and HACCP.
We specialize in the design, development and implementation of new automated technologies to standardize the disinfection process. The effectiveness of the production process is certified at all stages. This is the best guarantee that the product always meets the most stringent standards like electromagnetic compatibility and electrical safety.
MEDISYSTEM is the combination of our ”No Touch” patented high level Class I automatic medical device (CE marked/FDA registered) with micronebulizers from 2 to 4 nozzles, together with our own ready-to-use hydrogen peroxide Eco-friendly solutions.
Thanks to its international patent, the system disinfects by saturation of the most confined and hard to reach areas, also effective on smooth and porous surfaces. The result is simple (not corrosive/no residue).
Medisystem spectrum of effectiveness is higher than most competitive systems according to the latest EN standards, since it boats virucidal, bactericidal, mycobactericidal, fungicidal, yeasticidal, sporicidal and active also on biofilm. The system has high efficacy on both COVID-19 (SARS-CoV-2) and Monkeypox virus.
Standardization, traceability and automation of the process allow greater safety and exclude the possibility of human error, because it can be used regardless of operator intervention.
The system combination of micronebulization from <1 to 5 µm with H2O2 ensures that the disinfectant is applied correctly and for the necessary contact time, guaranteeing a high level reduction of the microorganisms.
IN VITRO TEST
| MICROORGANISM TESTED | STANDARD | ACTIVITY |
|---|---|---|
| Bacillus subtilis Bacillus cereus |
EN 14347:2005 (medical and veterinary areas, agricultural, industrial, domestic and institutional areas) |
SPORICIDE |
| Bacillus subtilis Bacillus cereus |
EN 13704:2018 (food, industrial, domestic and institutional areas) |
SPORICIDE |
| Pseudomonas aeruginosa Staphylococcus aureus Enterococcus hirae |
EN 13727:2015 (medical area) |
BACTERICIDE |
| Pseudomonas aeruginosa Staphylococcus aureus Enterococcus hirae MRSA Listeria monocytogenes Escherichia coli Salmonella enteritidis Legionella pneumophila |
EN 1276:2019 (food, industrial, domestic and institutional areas) |
BACTERICIDE |
| Mycobacterium terrae Mycobacterium avium |
EN 14348:2005 (medical area) |
MYCOBACTERICIDE |
| Poliovirus type 1 Adenovirus type 5 Murine Norovirus Vaccinia virus (SARS-CoV-2) |
EN 14476:2019 (medical area) |
VIRUCIDE |
| Adenovirus type 5 Murine Norovirus |
EN 16777:2019 (medical area) |
VIRUCIDE |
| Candida albicans Candida auris Aspergillus brasiliensis |
EN 13624:2013 (medical area) |
FUNGICIDE |
| Candida albicans Aspergillus brasiliensis Aspergillus niger DBS Collection |
EN 1650:2019 |
FUNGICIDE |
| Virus Influenza Type A-H1N1 | Test Lab | EFFICACY |
IN VIVO TEST
| MICROORGANISM TESTED | STANDARD | ACTIVITY |
|---|---|---|
| Pseudomonas aeruginosa Staphylococcus aureus Enterococcus hirae Escherichia coli Acinetobacter baumannii Proteus hauseri Bacillus subtilis Candida albicans Candida auris Aspergillus brasiliensis Mycobacterium avium Mycobacterium terrae |
EN 17272:2020 (medical and veterinary areas, food, industrial, domestic and institutional areas) |
SPORICIDE BACTERICIDE MYCOBACTERICIDE FUNGICIDE ON SURFACES |
| Bacillus subtilis Mycobacterium terrae |
AFNOR NF T72-190 | SPORICIDE MYCOBACTERICIDE ON SURFACES |
| Pseudomonas aeruginosa Staphylococcus aureus Enterococcus hirae |
EN 14561:2006 (medical area) |
BACTERICIDE ON SURFACES |
| Mycobacterium avium Mycobacterium terrae |
EN 14563:2009 (medical area) |
MYCOBACTERICIDE ON SURFACES |
| Aspergillus niger Candida albicans |
EN 14562:2006 (medical area) |
FUNGICIDE ON SURFACES |
| Clostridium difficile | Test Lab | EFFICACY |
| Serratia marcescens Klebsiella pneumoniae ESLB MDR Acinetobacter baumannii |
Test Lab | EFFICACY |
| Mycobacterium tuberculosis | Test Lab | EFFICACY |